Coronavirus Antibody Tests
Testing Options
Find out if you may have been infected by COVID-19.
Basic Antibody Test

Immune Response Antibody Test
Including Vaccine Response
Provides Qualitative N AB Results
This test measures the nucleocapsid antibody which is only present in those who have been exposed to and recovered from COVID-19.
This test measures the body’s spike protein levels which indicates how successful vaccine inoculation was at providing protective antibodies.
Want to know more about COVID-19 Test Options?
Get the answers to some of the most asked questions in our COVID-19 FAQs.
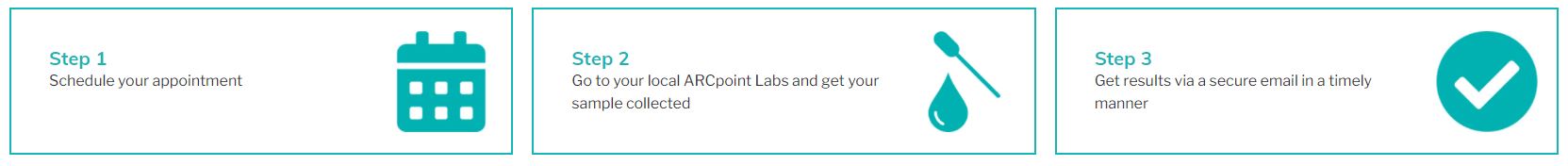
Testing is Simple And Quick

* Important Information
Please note that our tests have not been FDA cleared or approved, but they have been authorized by FDA under Emergency Use Authorizations (EUA’s) for use by authorized laboratories. Depending on the specific test type, these tests are authorized for the detection of SARS CoV-2, its proteins and/or its antibodies. These tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Please click below to see the specific FDA disclaimers and additional manufacturer information for each test and device used by ARCpoint or our laboratory partners.
