COVID-19 FAQs
Don’t Miss Your Flight
Whether traveling for work or just looking to relax on a nice getaway. Get your COVID test results quickly.
Beyond getting your passport ready and packing those essentials, you need a negative COVID-19 test before taking off for most international destinations. Don’t get stressed out by changing test regulations. Schedule your test now with ARCpoint Labs of Greenville, SC or Anderson, SC for a quick, convenient test.
Travel With Ease
Testing in the perfect window doesn’t have to be a hassle. Our catalog of COVID-19 tests will meet your destination’s requirements, and test results are designed to meet your wheels up.* Not sure what test your destination requires? Not a problem. Our industry experts are staying up-to-speed on changing regulations.
Planning a getaway should be an exciting time. You focus on packing, and let us handle the required COVID-19 testing.
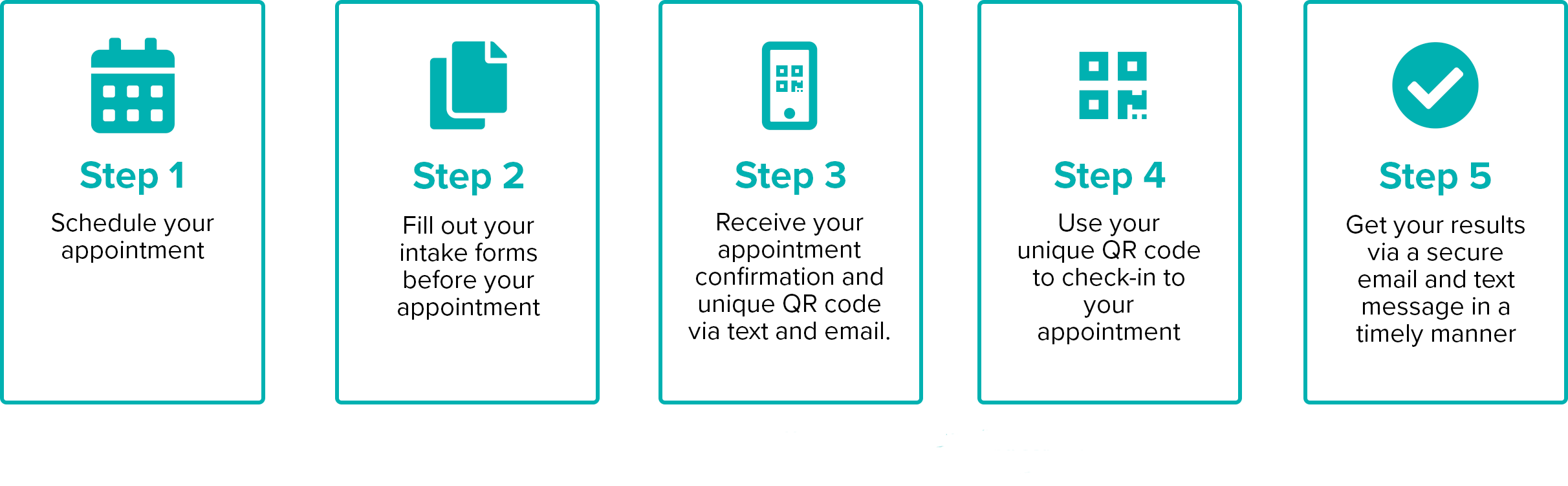
Testing is Simple And Quick
Want to know more about COVID-19 Test Options?
Get the answers to some of the most asked questions in our COVID-19 FAQs.

* Important Information
Please note that our tests have not been FDA cleared or approved, but they have been authorized by FDA under Emergency Use Authorizations (EUA’s) for use by authorized laboratories. Depending on the specific test type, these tests are authorized for the detection of SARS CoV-2, its proteins and/or its antibodies. These tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Please click below to see the specific FDA disclaimers and additional manufacturer information for each test and device used by ARCpoint or our laboratory partners.
