COVID-19 testing made quick and easy with ARCpoint Labs of Greenville, SC and Anderson, SC
Rapid COVID-19 Testing
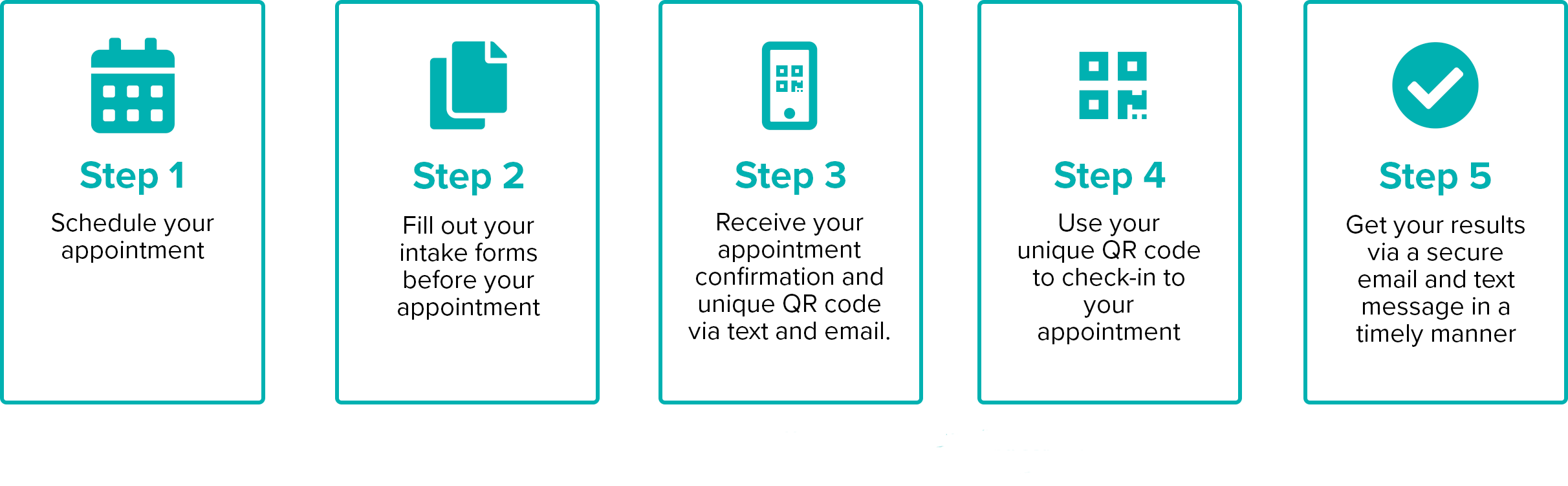
Getting COVID-19 test results back quickly can help you understand your risk of spreading the virus before seeing friends and family or returning to work. With a variety of testing options available, we can help determine which test is right for your situation and get your results back to you fast.
Testing is available at the following locations:
Greenville, SC
Anderson, SC
Need Help Deciding What Test You Need?
Do I have a current COVID-19 infection?
What if I have symptoms and think I might have COVID-19?
I may have been exposed, which test do I need?
Did I have a past COVID-19 infection?
I’m about to travel, what test do I need?
I’m about to have surgery, what test do I need?
Which testing do I need for my employees?

Whether traveling for work or just looking to relax on a nice getaway, you focus on packing, and let us handle the required COVID-19 testing. Our catalog of COVID-19 tests will meet your destination’s requirements, and test results are designed to meet your wheels up.
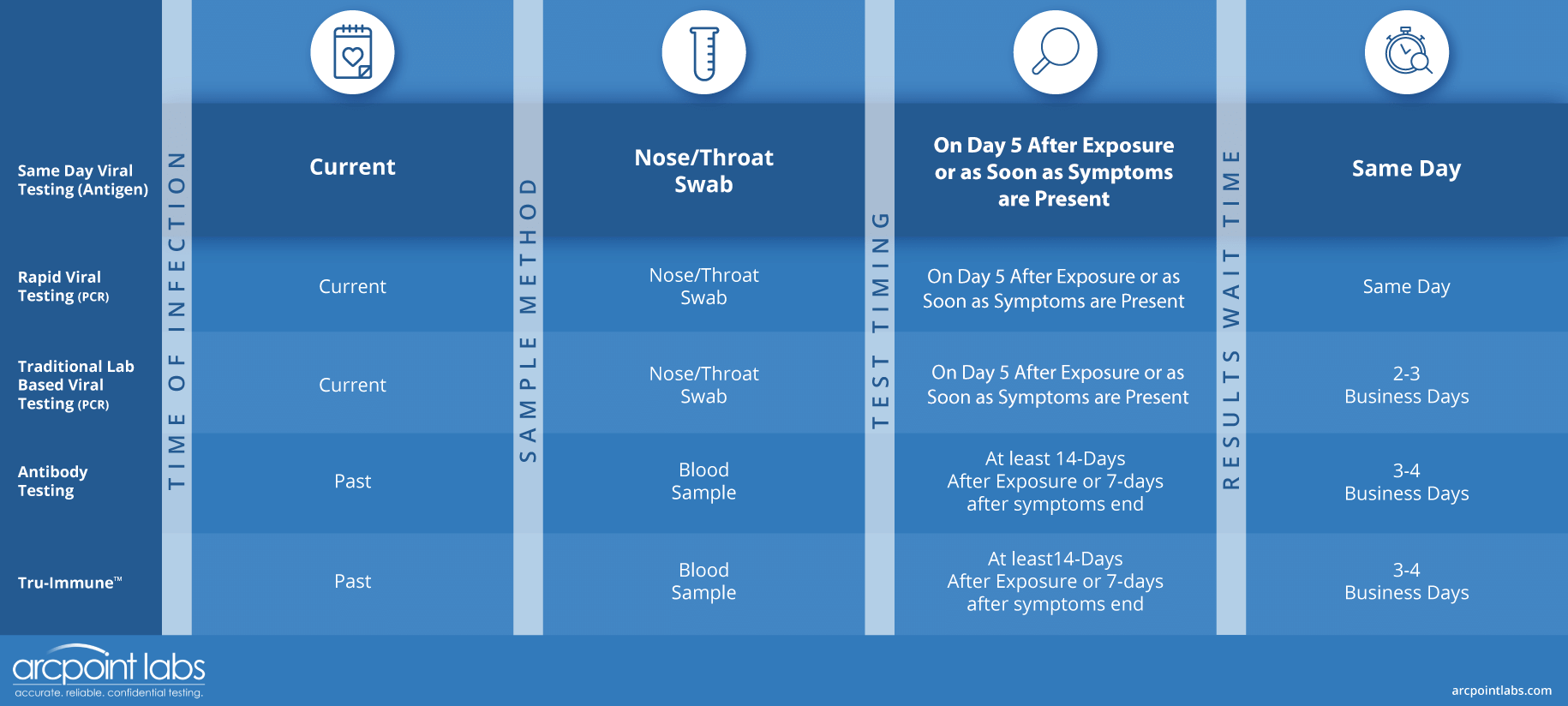
Same Day Viral Testing (Antigen)
The Rapid (Antigen) viral test uses a nasal/throat swab to detect a current infection of COVID-19 in your body. Best used 5 days after exposure, or sooner, if symptomatic, this test can help you determine if you need any further testing before returning to work, or if you should self-isolate.
Detects current infection
Same day results
Does not fit the requirements for most travel testing and select pre-surgical testing
Rapid Viral Testing (RT-PCR)
Detects current infection
Same day results
May fit the requirements for most travel testing and select pre-surgical testing
Traditional Lab Based Viral Testing (RT-PCR)
The Standard (RT-PCR) viral test uses a nasal/throat swab to detect a current infection of COVID-19 in your body. This test may be required before certain surgeries, returning to work, discontinuing self-isolation, and even traveling to certain countries.
Detects current infection
Results in 2-3 business days
Fits the requirements for travel and pre-surgical testing
Antibody Test (COVID-19 IgG/IgM)
Detects antibodies from past infection or vaccination
Results instantly
Requires a single drop of blood via finger prick
Comparing the Viral COVID-19 Testing Options
Did you know?
Our combo viral test can discover whether you have been infected with Influenza A or B, COVID-19, or RSV? Since the symptoms of all three viruses are so similar, it’s important to know which one you have.

* Important Information
Please note that our tests have not been FDA cleared or approved, but they have been authorized by FDA under Emergency Use Authorizations (EUA’s) for use by authorized laboratories. Depending on the specific test type, these tests are authorized for the detection of SARS CoV-2, its proteins and/or its antibodies. These tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Please click below to see the specific FDA disclaimers and additional manufacturer information for each test and device used by ARCpoint or our laboratory partners.
